5′-Nor-3-Deaza-1′,6′-Isoneplanocin, the Synthesis and Antiviral Study
Abstract
:1. Introduction
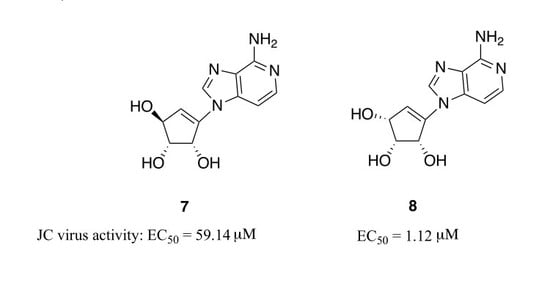
2. Results
3. Discussion
4. Materials and Methods
General Procedure of Ullmann Reaction
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers of Disease Control and Prevention. Available online: https://www.cdc.gov/vhf/ebola/index.html (accessed on 13 July 2020).
- Johns Hopkins University: Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/us-map (accessed on 13 July 2020).
- Yates, M.K.; Seley-Radtke, K.L. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antiviral Res. 2018, 154, 66–86. [Google Scholar]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antiviral Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Clercq, E.D. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 2019, 14, 3962–3968. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S.; Borchardt, R.T. S-Adenosyl-L-homocysteine hydrolase as a target for antiviral chemotherapy. J. Med. Chem. 1991, 34, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Seley, K.L.; Schneller, S.W. Does the anti-hepatitis B virus activity of (+)-5′-noraristeromycin exist in its 4′-epimer and 4′-deoxygenated derivatives? J. Med. Chem. 1998, 41, 2168–2170. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Q.; Schenller, S.W. Enantiomeric 3-deaza-1′,6′-isoneplanocin and its 3-bromo analogues: Synthesis by the Ullmann reaction and their antiviral properties. Bioorg. Med. Chem. Lett. 2016, 26, 928–930. [Google Scholar] [CrossRef]
- Yang, M.; Ye, W.; Schneller, S.W. Preparation of carbocyclic S-adenosylazamethionine accompanied by a practical synthesis of (−)-aristeromycin. J. Org. Chem. 2004, 69, 3993–3996. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Q.; Schneller, S.W. Both enantiomers of 6′-Isoneplanocin. Nucleos. Nucleot. Nucl. 2017, 36, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Smith, A. l-Like 3-deazaneplanocin analogues: Synthesis and antiviral properties. Bioorg. Med. Chem. Lett. 2019, 29, 126613. [Google Scholar] [CrossRef]
- Chen, Q.; Davidson, A. Synthesis, conformational study and antiviral activity of L-Like neplanocin derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Coleman, R.; Archer, A.; Hussein, I.; Bowlin, T.L.; Chen, Q.; Schneller, S.W. Enantiomeric 4′-Truncated 3-deaza-1′, 6′-isoneplanocins: Synthesis and antiviral properties including Ebola. Bioorg. Med. Chem. Lett. 2019, 29, 2480–2482. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Schneller, S.W.; Liu, C.; Jones, K.L.; Singer, T. 5′-Nor-3-Deaza-1′,6′-Isoneplanocin, the Synthesis and Antiviral Study. Molecules 2020, 25, 3865. https://doi.org/10.3390/molecules25173865
Chen Q, Schneller SW, Liu C, Jones KL, Singer T. 5′-Nor-3-Deaza-1′,6′-Isoneplanocin, the Synthesis and Antiviral Study. Molecules. 2020; 25(17):3865. https://doi.org/10.3390/molecules25173865
Chicago/Turabian StyleChen, Qi, Stewart W. Schneller, Chong Liu, Kathryn L. Jones, and Tyler Singer. 2020. "5′-Nor-3-Deaza-1′,6′-Isoneplanocin, the Synthesis and Antiviral Study" Molecules 25, no. 17: 3865. https://doi.org/10.3390/molecules25173865